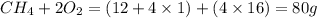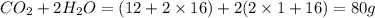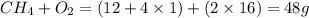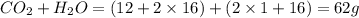Chemistry, 04.07.2019 14:00 ashleyheink3796
When methane, ch4, is combusted, it produces carbon dioxide, co2, according to the unbalanced equation: ch4 + o2 → co2 + h2o. write the balanced equation for this reaction, and explain how it is possible for 10 grams of methane fuel to burn and emit 27 grams of carbon dioxide. discuss whether or not this reaction obeys the law of conservation of mass.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 08:00
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
When methane, ch4, is combusted, it produces carbon dioxide, co2, according to the unbalanced equati...
Questions

Mathematics, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00

English, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00

Mathematics, 23.09.2021 14:00


Social Studies, 23.09.2021 14:00



Advanced Placement (AP), 23.09.2021 14:00

Mathematics, 23.09.2021 14:00



Mathematics, 23.09.2021 14:00




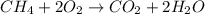
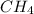 = 16 g/mole
= 16 g/mole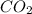 = 44 g/mole
= 44 g/mole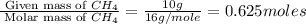
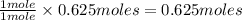 of
of