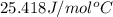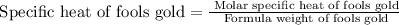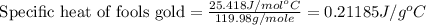Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Fools gold (iron pyrite) is made up of iron sulfide (fes2). it has a molar specific heat of 25.418j/...
Questions




History, 13.07.2019 17:00

Mathematics, 13.07.2019 17:00

History, 13.07.2019 17:00



Mathematics, 13.07.2019 17:00

Mathematics, 13.07.2019 17:00


History, 13.07.2019 17:00

Chemistry, 13.07.2019 17:00

History, 13.07.2019 17:00






 .
.