
Chemistry, 04.07.2019 20:30 kingje1477
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 2226.1kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How much heat is released upon converting one mole of steam (18.0 g) from 100.0 ∘c to water at 25.0 ∘c? show work and constants, trying to figure out how it works. only given the heat capacity for steam and water so try to only use that
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2
You know the right answer?
Calculate the standard enthalpy of combustion. the standard enthalpy of formation of sucrose is - 22...
Questions



History, 06.10.2019 04:30

History, 06.10.2019 04:30




English, 06.10.2019 04:30

Mathematics, 06.10.2019 04:30


Mathematics, 06.10.2019 04:30

Biology, 06.10.2019 04:30

Biology, 06.10.2019 04:30





Spanish, 06.10.2019 04:30

Mathematics, 06.10.2019 04:30

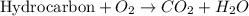
![\Delta H^o_{rxn}=\sum [n\times \Delta H_f^o_{(product)}]-\sum [n\times \Delta H_f^o_{(reactant)]}](/tpl/images/0051/4711/84379.png)
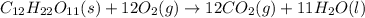
![\Delta H^o_{rxn}=[(12\times \Delta H_f^o_{(CO_2)})+(11\times \Delta H_f^o_{(H_2O)})]-[(1\times \Delta H_f^o_{(C_{12}H_{22}O_{11})})+(12\times \Delta H_f^o_{(O_2)})]](/tpl/images/0051/4711/fac1e.png)
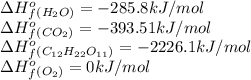
![\Delta H^o_{rxn}=[(12\times (-393.51))+(11\times (-285.8))]-[(1\times (-2226.1))+(12\times (0))]\\\\\Delta H^o_{rxn}=-5636.52kJ](/tpl/images/0051/4711/5e149.png)


