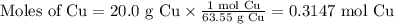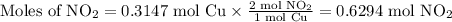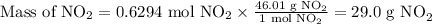Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3


Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
Cu(s) + 4hno3(aq)= cu(no3)(aq)+ 2no2(g) + 2h2o(l) how much no2 gas would be formed by the reaction...
Questions

Mathematics, 27.06.2019 11:30

Biology, 27.06.2019 11:30




Mathematics, 27.06.2019 11:30

Physics, 27.06.2019 11:30

Mathematics, 27.06.2019 11:30








History, 27.06.2019 11:30



Computers and Technology, 27.06.2019 11:30

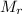 : 63.55 46.01
: 63.55 46.01