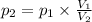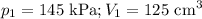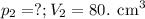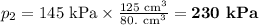Chemistry, 05.07.2019 02:00 cairolove228
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125 kpa. then the gas is compressed to a volume of 80. ml. what is the new pressure of the gas if the temperature is held at 25°c? (1) 69 kpa (3) 160 kpa (2) 93 kpa (4) 230 kpa the first person didn't make sense as p1v1 = p2v2 isnt the same thing as p1v1/v2 that literally doesn't make sense, so could someone me !

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 08:00
The goal of this experiment was to answer the question "what is the effect of a gas' temperature on its volume? " you formulated the hypothesis below. hypothesis: if a fixed amount of gas is heated, then the volume will increase because the heat will cause the molecules of gas to move faster and further apart. to test this hypothesis, you changed the of the gas between 0 and 100°c (273 and 373 k) and calculated the resulting of the gas.
Answers: 2
You know the right answer?
At 25°c, gas in a rigid cylinder with a movable piston has a volume of 145 ml and a pressure of 125...
Questions


Mathematics, 23.02.2021 14:00

Biology, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00



Mathematics, 23.02.2021 14:00

Spanish, 23.02.2021 14:00

Health, 23.02.2021 14:00

English, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Computers and Technology, 23.02.2021 14:00

Mathematics, 23.02.2021 14:00

Business, 23.02.2021 14:00

Health, 23.02.2021 14:00

Social Studies, 23.02.2021 14:00


Mathematics, 23.02.2021 14:00

Chemistry, 23.02.2021 14:00

Health, 23.02.2021 14:00

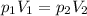
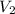 .
.