Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:10
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2



Chemistry, 23.06.2019 11:00
Nh4no3 n2o + 2h2o a chemist who is performing this reaction starts with 160.1 g of nh4no3. the molar mass of nh4no3 is 80.03 g/mol; the molar mass of water (h2o) is 18.01 g/mol. what mass, in grams, of h2o is produced?
Answers: 1
You know the right answer?
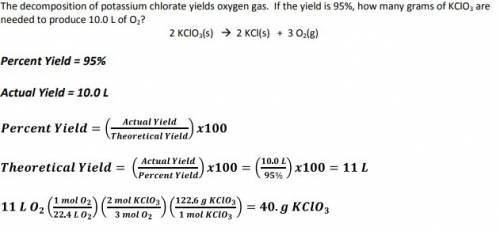
The decomposition of potassium chlorate yields oxygen gas. if the yield is 895%, how many grams of k...
Questions

Mathematics, 18.08.2021 09:00


Mathematics, 18.08.2021 09:00







Mathematics, 18.08.2021 09:00



Mathematics, 18.08.2021 09:00

Social Studies, 18.08.2021 09:00




Mathematics, 18.08.2021 09:00