
Chemistry, 05.07.2019 08:30 hdhdhd49jdhd
2na + 2h2o → 2naoh + h2 during a laboratory experiment, a certain quantity of sodium metal reacted with water to produce sodium hydroxide and hydrogen gas. what was the initial quantity of sodium metal used if 8.40 liters of h2 gas were produced at stp?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
2na + 2h2o → 2naoh + h2 during a laboratory experiment, a certain quantity of sodium metal reacted w...
Questions


Mathematics, 14.05.2021 19:20


History, 14.05.2021 19:20

English, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20



Mathematics, 14.05.2021 19:20



Mathematics, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20



Mathematics, 14.05.2021 19:20

Chemistry, 14.05.2021 19:20

Mathematics, 14.05.2021 19:20

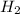 gas = 8.40 L
gas = 8.40 L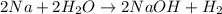
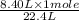 = 0.375 moles of
= 0.375 moles of 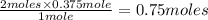 of Na metal
of Na metal


