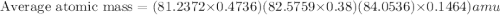Chemistry, 05.07.2019 08:30 aduncan3426
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47.36% of a standard sample. the second isotope, lc-83, has a mass of 82.5759 amu and makes up 38% of a standard sample. the third isotope, lc-85 has a mass of 84.0536 amu and makes up the remainder of the sample. what is the average atomic mass of this element?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible?
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
The element loncapium has three isotopes. the first lc-82 has a mass of 81.2372 amu and makes up 47....
Questions


Physics, 01.02.2021 21:40

Spanish, 01.02.2021 21:40

Mathematics, 01.02.2021 21:40



Mathematics, 01.02.2021 21:40



Mathematics, 01.02.2021 21:40

Mathematics, 01.02.2021 21:40

Mathematics, 01.02.2021 21:40

Social Studies, 01.02.2021 21:40

Chemistry, 01.02.2021 21:40

Mathematics, 01.02.2021 21:40

Mathematics, 01.02.2021 21:40




Mathematics, 01.02.2021 21:40

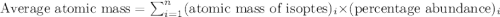 ....(1)
....(1)