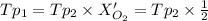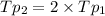Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

You know the right answer?
You have 4 moles of oxygen gas in a flask. 4 moles of helium gas is added. what happens to the total...
Questions

Mathematics, 04.08.2019 10:00



Health, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00


Chemistry, 04.08.2019 10:00

Health, 04.08.2019 10:00



Chemistry, 04.08.2019 10:00



Mathematics, 04.08.2019 10:00


Mathematics, 04.08.2019 10:00

Mathematics, 04.08.2019 10:00

English, 04.08.2019 10:00


English, 04.08.2019 10:00

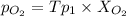 (
(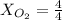 ) ( according to Dalton's law of partial pressure)
) ( according to Dalton's law of partial pressure)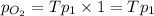 ....(1)
....(1)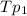 = Total pressure when only oxygen gas was present.
= Total pressure when only oxygen gas was present.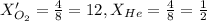
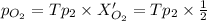
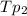 = Total pressure of the mixture.
= Total pressure of the mixture.