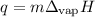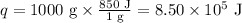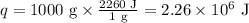Chemistry, 05.07.2019 10:30 dontcareanyonemo
Ethyl alcohol has a latent heat of vaporization of 850 j/g. water has a latent heat of vaporization of 2260 j/g. container a contains 1.0 kg of ethyl alcohol. container b contains 1.0 kg of water. both liquids are brought to a boil. if the same amount of heat is continuously added to each container, which liquid will boil away first? a ) water b ) ethyl alcohol c ) both will boil away at the same time d )boiling and condensation will happen at the same rate so neither liquid will boil away

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 16:00
Uranium can supply energy for the worlds electricity without admitting harmful greenhouse gases which of these statements best describes an outcome of uranium mining
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1
You know the right answer?
Ethyl alcohol has a latent heat of vaporization of 850 j/g. water has a latent heat of vaporization...
Questions

History, 13.10.2019 21:10

Advanced Placement (AP), 13.10.2019 21:10



Social Studies, 13.10.2019 21:10

Mathematics, 13.10.2019 21:10





Health, 13.10.2019 21:10


Physics, 13.10.2019 21:10

English, 13.10.2019 21:10




History, 13.10.2019 21:10