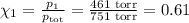Amixture of 60 mol % n-propylcyclohexane and 40 mol % n-propylbenzene is distilled through a simple distillation apparatus; assume that no fractionation occurs during the distillation. the boiling temperature is found to be 157 degrees celsius (760 torr) as the first small amount of distillate is collected. the standard vapor pressures of n-propylcyclohexane and n-propyl-benzene are known to be 769 torr and 725 torr, respectively, at 1567.3 degrees celsius. calculate the percentage of each of the two components in the first few drops of distillate.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1

Chemistry, 23.06.2019 03:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

You know the right answer?
Amixture of 60 mol % n-propylcyclohexane and 40 mol % n-propylbenzene is distilled through a simple...
Questions


World Languages, 24.04.2020 00:37


Mathematics, 24.04.2020 00:37

Mathematics, 24.04.2020 00:37

Mathematics, 24.04.2020 00:37

History, 24.04.2020 00:37

English, 24.04.2020 00:37

Mathematics, 24.04.2020 00:37





Mathematics, 24.04.2020 00:38

History, 24.04.2020 00:38


English, 24.04.2020 00:38


History, 24.04.2020 00:38

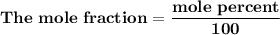
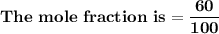
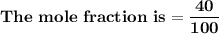
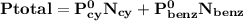
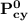 = n-propylcyclohexane vapor pressure
= n-propylcyclohexane vapor pressure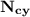 = n-propylcyclohexane mole fraction
= n-propylcyclohexane mole fraction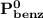 = n-propylbenzene vapor pressure
= n-propylbenzene vapor pressure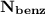 = n-propylbenzene mole fraction
= n-propylbenzene mole fraction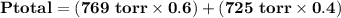
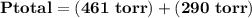

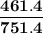

 and
and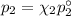
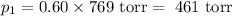
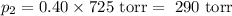
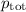 = p₁ + p₂= 461 torr + 290 torr = 751 torr
= p₁ + p₂= 461 torr + 290 torr = 751 torr