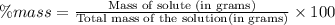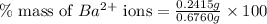Chemistry, 05.07.2019 11:30 pharadorvil04
Asample of 0.6760 g of an unknown compound containing barium ions (ba2+) is dissolved in water and treated with an excess of na2so4. if the mass of the baso4 precipitate formed is 0.4105 g, what is the percent by mass of ba in the original unknown compound?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2


Chemistry, 22.06.2019 23:30
To find the work done, the force exerted and distance moved are multiplied. a couch is moved twice before you are happy with its placement. the same force was used to move the couch both times. if more work is done the first time it is moved, what do you know about the distance it was moved? a) when more work was done, the couch was moved the same distance. b) when more work was done, the couch was moved less. c) when more work was done, the couch was moved further. d) when more work was done, the couch wasn't moved at all.
Answers: 1

You know the right answer?
Asample of 0.6760 g of an unknown compound containing barium ions (ba2+) is dissolved in water and t...
Questions

History, 26.01.2021 01:40


Mathematics, 26.01.2021 01:40


Spanish, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40


Physics, 26.01.2021 01:40


Mathematics, 26.01.2021 01:40


English, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40


Geography, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40

Mathematics, 26.01.2021 01:40



English, 26.01.2021 01:40

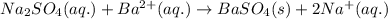
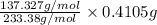 of Barium ions
of Barium ions