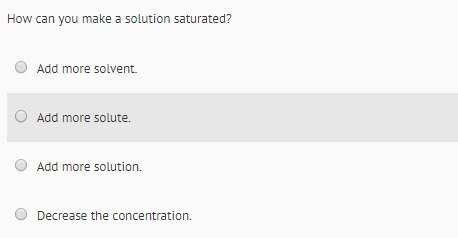
Chemistry, 05.07.2019 13:00 iicekingmann
Afictitious element q has two naturally occurring isotopes. the first isotope has an abundance of 10.00% and a mass of 39.06 amu and the second isotope of element q has an abundance of 90.00% and a mass of 42.14 amy. calculate the weighted atomic mass of element q to the nearest tenth. show work

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
Afictitious element q has two naturally occurring isotopes. the first isotope has an abundance of 10...
Questions

English, 27.10.2020 19:30

Geography, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30


English, 27.10.2020 19:30

Chemistry, 27.10.2020 19:30

English, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

English, 27.10.2020 19:30



Computers and Technology, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30

Mathematics, 27.10.2020 19:30



History, 27.10.2020 19:30


Mathematics, 27.10.2020 19:30