
Chemistry, 05.07.2019 14:00 amselah4955
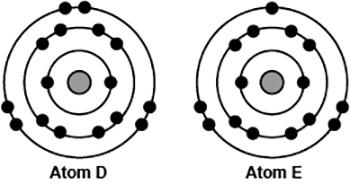
Brainliest + 20 points for best ! the image compares the arrangement of electrons in two different neutral atoms. which of the following best explains the position of the two atoms in the periodic table? a. both atoms have zeff of 8; therefore, atom d is to the right of atom e. b. both atoms have zeff of 2; therefore, atom d is below atom e in the same column. c. atom d has a zeff of 6 and is therefore to the right of atom e, which has a zeff of 5. d. atom d has a zeff of 14 and is therefore above atom e in the same column, which has a zeff of 13.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 23.06.2019 07:00
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 10:30
Fill in the blanks for the following statements: the rms speed of the molecules in a sample of h2 gas at 300 k will be times larger than the rms speed of o2 molecules at the same temperature, and the ratio µrms (h2) / µrms (o2) with increasing temperature. a not enough information is given to answer this question b sixteen, will not change c four, will not change d four, will increase e sixteen, will decrease
Answers: 2
You know the right answer?
Brainliest + 20 points for best ! the image compares the arrangement of electrons in two different...
Questions


English, 07.01.2021 01:00

English, 07.01.2021 01:00

Computers and Technology, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00

History, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


History, 07.01.2021 01:00

Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00


Mathematics, 07.01.2021 01:00




