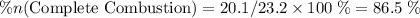Chemistry, 05.07.2019 18:00 amortalstardev
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incomplete combustion produces h2o and co, which not only reduces the efficiency of the engine using the fuel but is also toxic. in a certain test run, 1.000 gallon (gal) of octane is burned in an engine. the total mass of co, co2, and h2o produced is 11.53 kg. calculate the efficiency of the process; that is, calculate the fraction of octane converted to co2. the density of octane is 2.650 kg/gal.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Octane (c8h18) is a component of gasoline. complete combustion of octane yields h2o and co2. incompl...
Questions





History, 24.04.2020 19:48



Mathematics, 24.04.2020 19:48



Social Studies, 24.04.2020 19:48

Biology, 24.04.2020 19:48

History, 24.04.2020 19:48

English, 24.04.2020 19:48



Mathematics, 24.04.2020 19:48


Social Studies, 24.04.2020 19:48

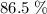 of octane had been converted to carbon dioxide CO₂.
of octane had been converted to carbon dioxide CO₂.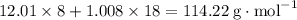
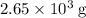 , which corresponds to
, which corresponds to 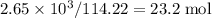 of octane.
of octane.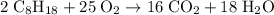
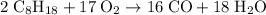
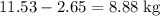 heavier than that of the octane supplied. Thus
heavier than that of the octane supplied. Thus 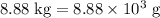 of oxygen were consumed in the combustion. There are
of oxygen were consumed in the combustion. There are 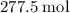 of oxygen molecules in
of oxygen molecules in 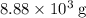 of oxygen.
of oxygen. (
(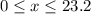 ). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal
). The number of moles of octane that had undergone incomplete combustion through the second equation would thus equal 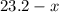 .
.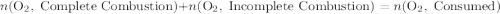
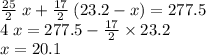
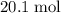 out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.
out of the 23.2 moles of octane had undergone complete combustion to produce carbon dioxide.