

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2


Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1
You know the right answer?
Which step in the free-radical chlorination of methane do you expect to be the most exothermic? att...
Questions



Social Studies, 20.11.2020 01:00

History, 20.11.2020 01:00


Arts, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00



Geography, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Physics, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00




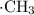 . The carbon-containing free radical would react with chlorine molecules to produce chloromethane and yet another chlorine free radical. This process can well repeat itself to chlorinate a significant number of methane molecules.
. The carbon-containing free radical would react with chlorine molecules to produce chloromethane and yet another chlorine free radical. This process can well repeat itself to chlorinate a significant number of methane molecules.

