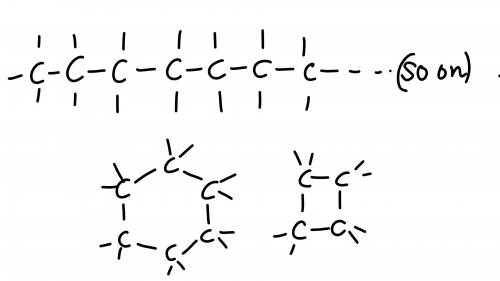Chemistry, 06.07.2019 05:30 sliverx201
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? available choices [a] each carbon atom can be stable with one, two, three, or four bonds because of how its valence electrons are arranged. [b] each carbon atom can bond with several other carbon atoms because of how many valence electrons it has. [c] each carbon atom donates its electrons to other atoms, including atoms of noble gases and halogens. [d] each carbon atom forms either double or triple bonds with surrounding hydrogen atoms. try your best, this is important, 25 points and brainliest answer if you get it right!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1
You know the right answer?
25 pointsquestion how does the structure of a carbon atom enable it to form large molecules? availab...
Questions

English, 04.12.2021 02:20



Mathematics, 04.12.2021 02:20

Mathematics, 04.12.2021 02:20

English, 04.12.2021 02:20

Biology, 04.12.2021 02:20


Social Studies, 04.12.2021 02:20

Biology, 04.12.2021 02:20



Social Studies, 04.12.2021 02:20


Mathematics, 04.12.2021 02:20





Chemistry, 04.12.2021 02:20