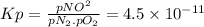Chemistry, 06.07.2019 06:30 NateTheBeast12
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automobile engine at 537°c is 4.5 × 10−11. n2(g) + o2(g) ⇌ 2no(g) (a) calculate the partial pressure of no under these conditions if the partial pressures of nitrogen and oxygen are 3.00 and 0.012 atm, respectively.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 06:30
Acertain atom has 22 protons and 19 electrons. this atom loses an electron. the net charge on the atom is now 4+1+01-4-. if this same atom with 22 protons and 19 electrons were to gain 3 electrons, the net charge on the atom would be 3+2+02-3-.
Answers: 1

You know the right answer?
The equilibrium constant (kp) for the formation of the air pollutant nitric oxide (no) in an automob...
Questions


History, 14.01.2020 04:31







Chemistry, 14.01.2020 04:31

Mathematics, 14.01.2020 04:31





Mathematics, 14.01.2020 04:31





Computers and Technology, 14.01.2020 04:31