
Chemistry, 03.12.2019 02:31 Nessakona1
Question 1 (true/false worth 4 points) (03.06 lc) an instantaneous dipole occurs when a molecule's moving electrons are briefly more concentrated in one place than another, causing the molecule to become temporarily polarized.
true false
question 2(multiple choice worth 4 points) (03.06 lc) what type of intermolecular force occurs between all substances?
covalent bonding
hydrogen bonding
ion-dipole force
london dispersion force
question 3(multiple choice worth 4 points) (03.06 mc) when comparing h2, nh3, o2, and ch4, which of the following statements is correct?
ch4 has the highest boiling point because it experiences dipole-dipole forces.
h2 has the strongest intermolecular forces because it has the lowest mass. nh3 has the highest boiling point because it experiences hydrogen bonding.
o2 has the strongest intermolecular force because it experiences london dispersion forces.
question 4(multiple choice worth 4 points) (03.06 mc)
the boiling points of diatomic halogens are compared in the table.
boiling points of diatomic halogens molecule boiling point
f2 −188 °c
cl2 −34 °c
br2 59 °c
i2 184 °c
which of the following statements best explains the trends in boiling points?
the atomic size increases down the group, and this decreases the strength of the intermolecular forces.
the total number of electrons decreases down the group, and this decreases the strength of the intermolecular forces.
the total number of electrons increases down the group, and this increases the strength of the intermolecular forces.
the chances of forming a permanent dipole increase down the group and this increases the strength of the intermolecular forces.
question 5(multiple choice worth 4 points) (03.06 mc) what is the strongest intermolecular force that occurs between molecules of co2?
dipole-dipole
induced dipoles
ionic bonding
london dispersion
asap 30 mark brainliest

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Nanotechnology, the field of trying to build ultrasmall structures one atom at a time, has progressed in recent years. one potential application of nanotechnology is the construction of artificial cells. the simplest cells would probably mimic red blood cells, the body's oxygen transporters. for example, nanocontainers, perhaps constructed of carbon, could be pumped full of oxygen and injected into a person's bloodstream. if the person needed additional oxygen-due to a heart attack perhaps, or for the purpose of space travel-these containers could slowly release oxygen into the blood, allowing tissues that would otherwise die to remain alive. suppose that the nanocontainers were cubic and had an edge length of 24 nanometers. part a part complete what is the volume of one nanocontainer? (ignore the thickness of the nanocontainer's wall.) express your answer using two significant figures. v v = 1.4ă—10â’20 l previous answers correct significant figures feedback: your answer 1.3824â‹…10â’20 = 1.382ă—10â’20 l was either rounded differently or used a different number of significant figures than required for this part. if you need this result for any later calculation in this item, keep all the digits and round as the final step before submitting your answer. part b suppose that each nanocontainer could contain pure oxygen pressurized to a density of 81 g/l . how many grams of oxygen could be contained by each nanocontainer?
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 12:30
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3
You know the right answer?
Question 1 (true/false worth 4 points) (03.06 lc) an instantaneous dipole occurs when a molecule's m...
Questions

Mathematics, 29.10.2020 21:50


English, 29.10.2020 21:50

Computers and Technology, 29.10.2020 21:50

English, 29.10.2020 21:50


Chemistry, 29.10.2020 21:50



Mathematics, 29.10.2020 21:50

English, 29.10.2020 21:50



Mathematics, 29.10.2020 21:50


History, 29.10.2020 21:50

Arts, 29.10.2020 21:50

Mathematics, 29.10.2020 21:50


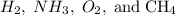 which of the following statements is correct?
which of the following statements is correct?
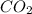 ?
?



