
Chemistry, 06.07.2019 10:00 omarabdellbast4334
1. the underlined portion of the chemical formula above is the and it represents a. coefficient; how many hydrogen atoms there are in one molecule b. subscript; how many hydrogen atoms there are in one molecule c. coefficient; how many water molecules there are d. subscript; how many water molecules there are 2. is 2h2 +o2 -> 2h2o. a balanced chemical equation? is it obeying the law of conservation of matter? a. yes, the equation is balanced. there are the same number of hydrogen (h) and oxygen (o) atoms on both sides of the equation. b. no, the equation is not balanced. the hydrogen (h) in the equation has less atoms represented on the products. c. no, the equation is not balanced. the oxygen (o) in the equation has less atoms represented on the products.
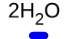

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
How heavy is thanos? a) 3000 lbs b) all of it c) the price of tea in china d) heavy enough
Answers: 2


Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
1. the underlined portion of the chemical formula above is the and it represents a. coefficient;...
Questions



History, 26.03.2020 16:35




Mathematics, 26.03.2020 16:35




Computers and Technology, 26.03.2020 16:35


Mathematics, 26.03.2020 16:35









