
Chemistry, 06.07.2019 13:00 briannagotfanz
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-gram sample undergoes decomposition, producing 283.4 grams of carbon, 31.7 grams of hydrogen, and 377.4 grams of oxygen. what is the molecular formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 10:30
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Acompound is used as a food additive. the compound has a molar mass of 176.124 grams/mole. a 692.5-g...
Questions


Mathematics, 23.03.2021 22:50




Biology, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50


Mathematics, 23.03.2021 22:50

Mathematics, 23.03.2021 22:50

Social Studies, 23.03.2021 22:50

English, 23.03.2021 22:50

Biology, 23.03.2021 22:50



History, 23.03.2021 22:50



Geography, 23.03.2021 22:50

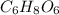 .
.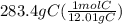
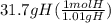
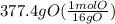
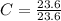 = 1
= 1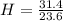 = 1.33
= 1.33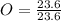 = 1
= 1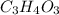 .
.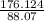 = 2
= 2


