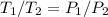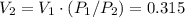Chemistry, 06.07.2019 17:00 miguel454545
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increased from 170 to 5: 40 the original volume of gas is one leader assume the temperature and number of particles are consistent what is the new volume

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 13:00
What did the experiments of scientists after john dalton reveal about his atomic theory?
Answers: 1
You know the right answer?
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increas...
Questions


Business, 26.12.2019 02:31

Mathematics, 26.12.2019 02:31


Geography, 26.12.2019 02:31


Social Studies, 26.12.2019 02:31


Chemistry, 26.12.2019 02:31

English, 26.12.2019 02:31





History, 26.12.2019 02:31





 Initial Pressure
Initial Pressure  Final Pressure
Final Pressure