What does the pauli exclusion principle state?
a) electrons will be added to an atom a...

What does the pauli exclusion principle state?
a) electrons will be added to an atom at the lowest possible level or sublevel.
b) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.
c) an orbital can contain two electrons only if all other orbitals at that sublevel contain at least one electron.
d) two electrons occupy the same orbital only if they have opposite spins.
e) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3


Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 08:00
How many distinct monochlorinated products, including stereoisomers, can result when the alkane below is heated in the presence of cl2? 3 4 5 6 7?
Answers: 3
You know the right answer?
Questions

History, 20.08.2019 10:00

World Languages, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00

History, 20.08.2019 10:00

Geography, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00



Social Studies, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00

Health, 20.08.2019 10:00

Mathematics, 20.08.2019 10:00




Mathematics, 20.08.2019 10:00


Mathematics, 20.08.2019 10:00

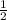 or
or  .
.

