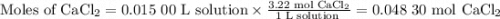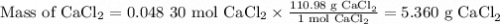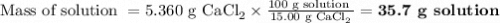Chemistry, 07.07.2019 03:00 staz13wiggins
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solution that is 15.00% cacl2 by mass, what is the final mass of the new solution. the density of water is exactly 1.00 g/ml. assume that the density of the cacl2 solution is also exactly 1.00 g/ml.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Amixture of gaseous sulfur dioxide and oxygen are added to a reaction vessel and heated to 1000 k where they react to form so3(g). if the vessel contains 0.669 atm so2(g), 0.395 atm o2(g), and 0.0851 atm so3(g) after the system has reached equilibrium, what is the equilibrium constant kp for the reaction: 2 so2(g) o2(g) ⇌ 2 so3(g)
Answers: 3

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1


Chemistry, 22.06.2019 19:30
Astudent conducts an experiment to determine how the amount of water given to a plant affects its growth. what is the independent variable for this experiment?
Answers: 1
You know the right answer?
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solut...
Questions

English, 30.08.2019 02:00


Mathematics, 30.08.2019 02:00

English, 30.08.2019 02:00

Biology, 30.08.2019 02:00




History, 30.08.2019 02:00







Mathematics, 30.08.2019 02:00

Social Studies, 30.08.2019 02:00

Health, 30.08.2019 02:00