
Chemistry, 07.07.2019 03:00 TheHomieJaay3092
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2al(s)+6hcl(aq)-> 2alcl3(aq) +3h2(g) what mass of al(s) is required to produce 513.0 ml of h2(g) at stp?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 07:00
Introduction of drugs into the gastrointestinal tract is a form of administration. a. enteral b. topical c. parenteral d. inhalation
Answers: 1
You know the right answer?
When aluminum is placed in concentrated hydrochloric acid, hydrogen gas is produced. 2al(s)+6hcl(aq)...
Questions




History, 26.05.2020 23:04

Mathematics, 26.05.2020 23:04

Mathematics, 26.05.2020 23:04

Mathematics, 26.05.2020 23:04


Mathematics, 26.05.2020 23:04

Mathematics, 26.05.2020 23:04

English, 26.05.2020 23:04

Social Studies, 26.05.2020 23:04


History, 26.05.2020 23:04



Health, 26.05.2020 23:04

History, 26.05.2020 23:04


Mathematics, 26.05.2020 23:04

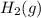 at STP = 513 ml = 0.513 L ( 1 L = 1000 ml )
at STP = 513 ml = 0.513 L ( 1 L = 1000 ml )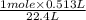 moles of
moles of 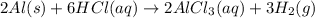
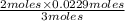 = 0.0153 moles
= 0.0153 moles

