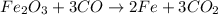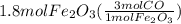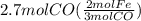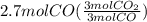Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co, how many moles of each product are formed? 5.4 moles fe and 1.8 moles co2 2.7 moles fe and 0.9 moles co2 3.6 moles fe and 5.4 moles co2 1.8 moles fe and 2.7 moles co2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 12:20
Consider the reaction of a(g) + b(g) + c(g) => d(g) for which the following data were obtained: experiment initial [a], mol/l initial [b], mol/l initial [c], mol/l initial rate, mol/l.s 1 0.0500 0.0500 0.0100 6.25 x 10^-3 2 0.100 0.0500 0.0100 2.50 x 10^-2 3 0.100 0.100 0.0100 1.00 x 10^-1 4 0.0500 0.0500 0.0200 6.25 x 10^-3 what is the rate law for the reaction?
Answers: 3

Chemistry, 22.06.2019 16:50
Assuming complete dissociation of the solute, how many grams of kno3 must be added to 275 ml of water to produce a solution that freezes at -14.5 c? the freezing point for pure water is 0.0 c and k_f is equal to 1.86 c/m
Answers: 3
You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co,...
Questions

English, 15.10.2019 19:00

Mathematics, 15.10.2019 19:00



History, 15.10.2019 19:00


Physics, 15.10.2019 19:00






English, 15.10.2019 19:00

History, 15.10.2019 19:00






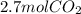 .
.