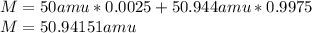Chemistry, 07.07.2019 07:00 mariahisawsome1
Plsss how do anion formation and valence electrons relate? a) atoms gain valence electrons to form anions. b) atoms yield valence electrons to form anions. c) atoms donate valence electrons to form anions. d) atoms relinquish valence electrons to form anions. vanadium has two naturally occuring isotopes - vanadium-50 and vanadium-51. predict the isotopic mass of vanadium-50 given that vanadium-50 has an abundance of 0.250% and that vanadium-51 has an abundance of 99.750% and a mass of 50.944 amu. a) 49.944 amu b) 62.558 amu c) 63.303 amu d) 5094.151 amu scientists use the emission spectra of elements to detect a) explosives in luggage. b) cracks in support structures, like bridges. c) the possibility of an earthquake occurrence. d) elements in clouds of gas and dust in deep space stars, such as our sun, use fusion to combine hydrogen atoms into helium atoms, and in the process, create energy. as massive stars use the last of their helium fuel, they begin to collapse and temperatures climb high enough to fuse other heavier elements. as elements increase in atomic number, the amount of energy required for fusion to occur also increases. nickel represents the heaviest element that can be produced by fusion due to to the net energy requirements. two atoms of could combine by fusion in order to create nickel. a) hydrogen b) nitrogen c) oxygen d) silicon

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 21.06.2019 20:30
Which of the following pairs of elements belong to the same groupa. h and he b. li and bec. c and pb d. ga and ge
Answers: 1

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 20:40
Select the correct value for the indicated bond angle in each of the compounds. o−o−oo−o−o angle of o3 90° 109.5° < 109.5° 120° < 120° 180° f−b−ff−b−f angle of bf3 180° < 109.5° < 120° 120° 109.5° 90° f−o−ff−o−f angle of of2 < 120° 120° 90° 109.5° 180° < 109.5° cl−be−clcl−be−cl angle of becl2 90° 109.5° 180° 120° < 109.5° < 120° f−p−ff−p−f angle of pf3 90° 109.5° < 109.5° 180° 120° < 120° h−c−hh−c−h angle of ch4 90° < 109.5° 180° 120° < 120° 109.5°
Answers: 1
You know the right answer?
Plsss how do anion formation and valence electrons relate? a) atoms gain valence electrons to form...
Questions

Mathematics, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31



Social Studies, 30.12.2019 04:31


Mathematics, 30.12.2019 04:31



English, 30.12.2019 04:31


Mathematics, 30.12.2019 04:31



Chemistry, 30.12.2019 04:31

Mathematics, 30.12.2019 04:31


Mathematics, 30.12.2019 04:31