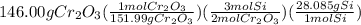Chemistry, 07.07.2019 13:30 lazavionadams81
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3si(> 4cr(l) + 3sio2 (s) the reaction is begun with 167.00 g of si and 146.00 g of cr2o3. how many grams of the excess reactant are left after the reaction is complete? i found which one was the l. r but i can't figure out how to find the excess amount of the e. r. will be greatly

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 23:10
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3


Chemistry, 23.06.2019 10:00
Which of the following reasons best explains why a scientist would want to replicate gregor mendel's pea plant experiment? a. to discover new aspects of the natural world b. to test the predictions of current theories c. to explain recently observed phenomena d. to test the conclusions of prior investigations
Answers: 1
You know the right answer?
The reaction of cr2o3 with silicon metal at high temperatures will make chromium metal. 2cro3(s) + 3...
Questions

Mathematics, 19.11.2020 17:20

Mathematics, 19.11.2020 17:20

Business, 19.11.2020 17:20

Biology, 19.11.2020 17:20

Mathematics, 19.11.2020 17:20

Mathematics, 19.11.2020 17:20





Mathematics, 19.11.2020 17:20




English, 19.11.2020 17:20



History, 19.11.2020 17:20


Mathematics, 19.11.2020 17:20

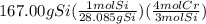
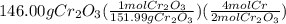
 gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.
gives the less moles of the product and so it is limiting reactant and hence the excess reactant is Si.