
Chemistry, 07.07.2019 15:00 gdominguez2005
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a solution at a ph = 2 than in a solution at a ph = 6. find the concentration of h+ ions at a ph = 2 and at a ph = 6 in table b. then divide the concentration of h+ ions at a ph = 2 by the of h+ ions at a ph = 6. record your answer in table c. what is the concentration of h+ ions at a ph = 2? mol/l what is the concentration of h+ ions at a ph = 6? mol/l how many more h+ ions are there in a solution at a ph = 2 than in a solution at a ph = 6? need asap give brainliest

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a...
Questions


Mathematics, 14.05.2021 19:30


English, 14.05.2021 19:30





Health, 14.05.2021 19:30



Mathematics, 14.05.2021 19:30

Chemistry, 14.05.2021 19:30

Mathematics, 14.05.2021 19:30




English, 14.05.2021 19:30


Mathematics, 14.05.2021 19:30

![pH=-log[H⁺]](/tpl/images/0062/1602/22ab6.png)
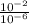 = 10⁴
= 10⁴

