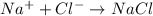It is difficult to break the ionic bonds in a compound because of a. good conductivity between ions. b. small distance between the ions. c. strong attraction between ions. ionic compounds are solid at room temperature because they a. high melting points b. ion with similar charges c. no valence electrons

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2


Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
It is difficult to break the ionic bonds in a compound because of a. good conductivity between ion...
Questions




Biology, 02.02.2020 12:48

Mathematics, 02.02.2020 12:48


Physics, 02.02.2020 12:48


Mathematics, 02.02.2020 12:48

Mathematics, 02.02.2020 12:48


Chemistry, 02.02.2020 12:48

Social Studies, 02.02.2020 12:48

Mathematics, 02.02.2020 12:48






![Na:11:[Ne]3s^1](/tpl/images/0062/6064/98162.png)
![Na^+:10:[Ne]](/tpl/images/0062/6064/a96fa.png)
![Cl:17:[Ne]3s^23p^5](/tpl/images/0062/6064/bf183.png)
![Cl^-:18:[Ne]3s^23p^6](/tpl/images/0062/6064/1914b.png)