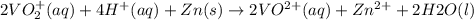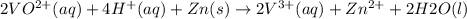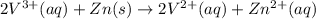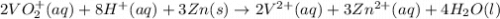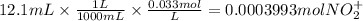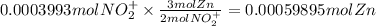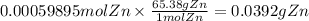Chemistry, 07.07.2019 19:30 tladitidimatso1783
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction from +5 to +4: 2 vo2+(aq) + 4 h +(aq) + zn(s) → 2 vo2+(aq) + zn2+(aq) + 2 h2o(l) reduction from +4 to +3: 2 vo2+(aq) + zn(s) + 4 h +(aq) → 2 v3+(aq) + zn2+(aq) + 2 h2o(l) reduction from +3 to +2: 2 v3+(aq) + zn(s) → 2 v2+(aq) + zn2+(aq) if you had 12.1 ml of a 0.0033 m solution of vo2+(aq), how many grams of zn metal would be required to completely reduce the vanadium?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1
You know the right answer?
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction fro...
Questions

Biology, 23.10.2019 18:00


Spanish, 23.10.2019 18:00




Computers and Technology, 23.10.2019 18:00



Physics, 23.10.2019 18:00







Computers and Technology, 23.10.2019 18:00

Computers and Technology, 23.10.2019 18:00