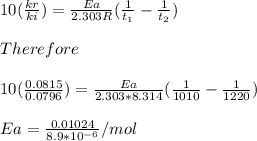Chemistry, 07.07.2019 21:00 madisontrosclair2
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. the decomposition of nitric oxide (no) to n2 and o2 is second order with a rate constant of 0.0796 m−1⋅s−1 at 737∘c and 0.0815 m−1⋅s−1 at 947∘c. you may want to reference (page) section 14.5 while completing this problem. part a calculate the activation energy for the reaction. express the activation energy in kilojoules per mole to three significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1


Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions

English, 18.12.2021 14:00

Arts, 18.12.2021 14:00


History, 18.12.2021 14:00

Mathematics, 18.12.2021 14:00



History, 18.12.2021 14:00


Mathematics, 18.12.2021 14:00


Social Studies, 18.12.2021 14:00





Mathematics, 18.12.2021 14:00