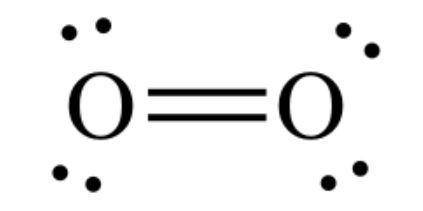Chemistry, 07.07.2019 23:00 etowens5604
When two oxygen atoms bond together in o2, what type of covalent bond do they form? a double bond, because they overlap orbitals to share one pair of electrons. a double bond, because they overlap orbitals to share two pairs of electrons. a single bond, because they overlap orbitals to share one pair of electrons. a single bond, because they overlap orbitals to share two pairs of electrons.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
When two oxygen atoms bond together in o2, what type of covalent bond do they form? a double bond,...
Questions



Mathematics, 05.11.2020 18:40



Business, 05.11.2020 18:40


Computers and Technology, 05.11.2020 18:40

Mathematics, 05.11.2020 18:40


Mathematics, 05.11.2020 18:40

History, 05.11.2020 18:40



Computers and Technology, 05.11.2020 18:40

English, 05.11.2020 18:40




Mathematics, 05.11.2020 18:40

![[Ne]3s^23p^4](/tpl/images/0063/4088/9a0fd.png)