Chemistry, 08.07.2019 00:30 bestgamer7373
Wheen 56j of heat are added to 11g of liquid, its temperature rises from 10.4 degrees celsius to 12 degrees celsius. what is the heat capacity of the liquid

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 22.06.2019 17:00
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2

Chemistry, 22.06.2019 18:30
Read the claim. breakfast is an important meal. it jump starts the body’s process of using calories to break down food. appetite can decrease with age, but going too long without eating causes metabolism to slow down. current research shows that incorporating legumes such as lentils and chickpeas into meals boosts metabolism for twenty-four hours. who might benefit from this claim? people who have a fast metabolism stores that sell exercise equipment people who take vitamin supplements grocery stores that sell legumes
Answers: 1
You know the right answer?
Wheen 56j of heat are added to 11g of liquid, its temperature rises from 10.4 degrees celsius to 12...
Questions

English, 31.10.2020 03:40

English, 31.10.2020 03:40



English, 31.10.2020 03:40

English, 31.10.2020 03:40

Mathematics, 31.10.2020 03:40


Mathematics, 31.10.2020 03:40

Mathematics, 31.10.2020 03:40


Chemistry, 31.10.2020 03:40

Mathematics, 31.10.2020 03:40

Mathematics, 31.10.2020 03:40

English, 31.10.2020 03:40

Biology, 31.10.2020 03:40

Advanced Placement (AP), 31.10.2020 03:40

Mathematics, 31.10.2020 03:40

Social Studies, 31.10.2020 03:40

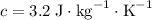
 Mass being heated
Mass being heated  Temperature change
Temperature change 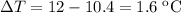 which is the same as
which is the same as  .
.