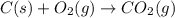The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn all the charcoal and the grill has a mass of 30.0 kg. what is the mass in kg of the gases given off? (assume that the charcoal is pure carbon solid and that it burns completely in oxygen). the mass of carbon burnt = 2kg = 2000g moles of carbon burnt = 2000/12 => moles of co2 produced = 2000/12 => mass of co2 produced = 2000/12*44 = 2 333.33 g = 2.33 kg in the answer, where did the 44 come from?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 23.06.2019 09:30
Where are the noble gases located in the periodic table? a. in the center b. on the left side c. in the upper right corner d. on the far right side
Answers: 1

Chemistry, 23.06.2019 18:30
Calcium hydroxide and hydrochloric acid react to form calcium chloride and water as shown in the chemical reaction. if the chemicals are present in exactly the correct ratios to fully use all of the ingredients, how many moles of water would be formed from 5 moles of hcl? (oh)2 + ? +
Answers: 1
You know the right answer?
The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn a...
Questions

Mathematics, 14.12.2021 05:40

Mathematics, 14.12.2021 05:40

Computers and Technology, 14.12.2021 05:40

Mathematics, 14.12.2021 05:40

Mathematics, 14.12.2021 05:40

English, 14.12.2021 05:40

Mathematics, 14.12.2021 05:40


Chemistry, 14.12.2021 05:40



Mathematics, 14.12.2021 05:40


SAT, 14.12.2021 05:40

Business, 14.12.2021 05:40


Mathematics, 14.12.2021 05:40


Mathematics, 14.12.2021 05:40

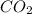 .
.