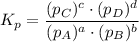Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2


Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3
You know the right answer?
Consider the equilibrium, n2(g) + 2o2(g) < > n2o4(g). calculate the equilibrium constant, kpi...
Questions

Chemistry, 12.10.2019 05:50





Mathematics, 12.10.2019 05:50




Social Studies, 12.10.2019 05:50



Social Studies, 12.10.2019 05:50


Spanish, 12.10.2019 05:50

History, 12.10.2019 05:50

Mathematics, 12.10.2019 05:50

History, 12.10.2019 05:50