Chemistry, 08.07.2019 11:00 Brainly264
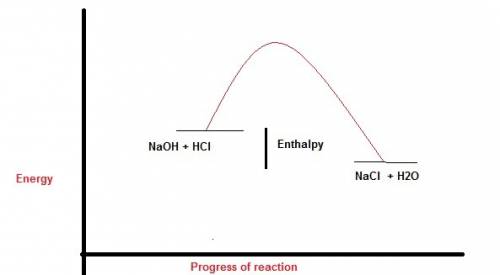
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-54 kj a. gambarlah diagram tingkat energi untuk reaksi tersebut b. berapakah perubahan entalpi jika 100 ml naoh 1 m ? c. berapakah perubahan entalpi jika 10 ml hcl 1 m direaksikan dengan 20 ml naoh 1 m ?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 03:30
Ineed pls urgent 1-20 in order and fully detail step my step.
Answers: 1
You know the right answer?
Perhatikan persamaan termokimia berikut. hcl (aq) + naoh (aq) -> nacl (aq) + h2o (l). delta h=-5...
Questions




Chemistry, 25.12.2021 03:30



Mathematics, 25.12.2021 03:30

Social Studies, 25.12.2021 03:30

Business, 25.12.2021 03:30

Social Studies, 25.12.2021 03:30

Social Studies, 25.12.2021 03:30







Social Studies, 25.12.2021 03:30