

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2


Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
We are given an unknown antacid pill and through the procedure in this lab we determine its cost eff...
Questions


Law, 24.04.2021 18:30

Advanced Placement (AP), 24.04.2021 18:30


Mathematics, 24.04.2021 18:30


History, 24.04.2021 18:30



English, 24.04.2021 18:30

Business, 24.04.2021 18:30


Mathematics, 24.04.2021 18:30

Mathematics, 24.04.2021 18:30


English, 24.04.2021 18:30




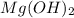 . An antacid pill is used in the case of acidity to neutralize the excessive acid content formed in stomach.
. An antacid pill is used in the case of acidity to neutralize the excessive acid content formed in stomach.


