
Chemistry, 08.07.2019 15:30 littlebrain2672
Calculate the concentrations of iron(iii) ions and scn- ions when 5.00 ml of 0.002m iron(iii) nitrate is mixed with 4.00 ml of 0.002 m nascn and dilute nitric acid is added to bring the total volume of the solution to 20.0 ml. what is the concentration of iron(iii) ions?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1


Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1
You know the right answer?
Calculate the concentrations of iron(iii) ions and scn- ions when 5.00 ml of 0.002m iron(iii) nitrat...
Questions




Mathematics, 28.01.2021 05:30

Mathematics, 28.01.2021 05:30

Mathematics, 28.01.2021 05:30

Mathematics, 28.01.2021 05:30

Mathematics, 28.01.2021 05:30

Social Studies, 28.01.2021 05:30




Mathematics, 28.01.2021 05:30

Geography, 28.01.2021 05:30

Geography, 28.01.2021 05:30


Mathematics, 28.01.2021 05:30

English, 28.01.2021 05:30


Mathematics, 28.01.2021 05:30

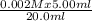 = 5 x 10⁻⁴
= 5 x 10⁻⁴

