
Chemistry, 08.07.2019 20:30 harlem3166
The specific heat of a substance is 0.990j/g. what is the value in kcal/mg?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 02:30
what is your question? collegechemistry 5+3 pts in november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3

Chemistry, 23.06.2019 06:20
What is the magnitude of the force of gravity between to 1000 kg cars which are separated by distance of 25. 0 km on an interstate highway? the force between the two cars will be what
Answers: 3

Chemistry, 23.06.2019 07:30
Using this reversible reaction, answer the questions below: n2o4 2no2 (colorless) (reddish-brown) -as the temperature increased, what happened to the n2o4 concentration? -was the formation of reactants or products favored by the addition of heat? -which reaction is exothermic? right to left or left to right? -if the change of enthalpy of this reaction when proceeding left to right is 14 kcal, which chemical equation is correct? n2o4 2no2 + 14 kcal n2o4 2no2, hr = +14 kcal n2o4 + 14 kcal 2no2 n2o4 2no2, hr = -14 kcal
Answers: 1

You know the right answer?
The specific heat of a substance is 0.990j/g. what is the value in kcal/mg?...
Questions

Physics, 06.09.2021 14:00


Mathematics, 06.09.2021 14:00

Advanced Placement (AP), 06.09.2021 14:00

Social Studies, 06.09.2021 14:00





Mathematics, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00

Geography, 06.09.2021 14:00

Physics, 06.09.2021 14:00

Physics, 06.09.2021 14:00



Geography, 06.09.2021 14:00

Physics, 06.09.2021 14:00

Mathematics, 06.09.2021 14:00

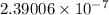
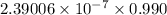 ) kcal/mg
) kcal/mg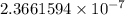 kcal/mg
kcal/mg

