
Chemistry, 08.07.2019 21:00 khadythiam6957
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87 mol of phosphorus and 3.86 mol of oxygen are combined. (assume 100% yield). a) write a balanced equation for the reaction. b) what is the limiting reactant? c) how many miles of excess reactant remain after the reaction is complete?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87...
Questions

Mathematics, 21.05.2020 22:10


Mathematics, 21.05.2020 22:10


Mathematics, 21.05.2020 22:10

Mathematics, 21.05.2020 22:10

Biology, 21.05.2020 22:10

Mathematics, 21.05.2020 22:10

English, 21.05.2020 22:10



Mathematics, 21.05.2020 22:10


Mathematics, 21.05.2020 22:10


Social Studies, 21.05.2020 22:10

Mathematics, 21.05.2020 22:10

Mathematics, 21.05.2020 22:10

Mathematics, 21.05.2020 22:10

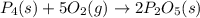
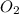 .
.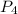 is 0.94 moles.
is 0.94 moles.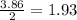 moles of
moles of 


