

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 18:00
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2
You know the right answer?
An unknown metal with a mass of 28g absorbs 58j of heat. it’s temperature rises from 31.1 c to 39.9...
Questions

Geography, 02.10.2019 04:30

Business, 02.10.2019 04:30



Mathematics, 02.10.2019 04:30

English, 02.10.2019 04:30


Mathematics, 02.10.2019 04:30

Mathematics, 02.10.2019 04:30


History, 02.10.2019 04:30

English, 02.10.2019 04:30

English, 02.10.2019 04:30

History, 02.10.2019 04:30

Mathematics, 02.10.2019 04:30



Mathematics, 02.10.2019 04:30

Mathematics, 02.10.2019 04:30

English, 02.10.2019 04:30

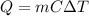 -(1)
-(1) is change in temperature.
is change in temperature.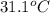 (given)
(given)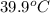 (given)
(given)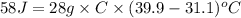
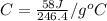
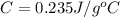
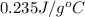 .
.

