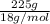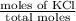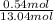Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 23.06.2019 07:10
1) a light bulb takes in 30 of energy per second. it transfers 3j as use energy. calculate the efficiency. second. it transfers 3j as useful light energy and 27j as heat energy. calculate the efficiency
Answers: 1

Chemistry, 23.06.2019 10:30
4al + 3o2 → 2al2o3 what does the "3" in front of o2 stand for? a) it indicates that there are 5 oxygen atoms after you add the coefficient and the subscript. b) it indicates that that there are are total of 6 oxygen atoms all bonded together as a single molecule. c) it indicates that there are 3 oxygen molecules chemically bonded to each other in the reaction. d) it indicates that there are 3 separate oxygen molecules in the reaction.
Answers: 2

Chemistry, 23.06.2019 11:00
The image below shows a weather service map.. i’m not sure if is correct
Answers: 2
You know the right answer?
Asolution was prepared by dissolving 40.0 g of kcl in 225 g of water. part a calculate the mass perc...
Questions

Mathematics, 11.11.2019 23:31


Mathematics, 11.11.2019 23:31


History, 11.11.2019 23:31


Geography, 11.11.2019 23:31

History, 11.11.2019 23:31



Mathematics, 11.11.2019 23:31



Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31

Mathematics, 11.11.2019 23:31



Biology, 11.11.2019 23:31

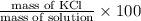
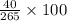
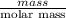
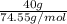
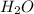 =
=