Chemistry, 18.11.2019 04:31 robert7248
Deuterium, 2h (2.0140 u), is sometimes used to replace the principal hydrogen isotope 1h in chemical studies. the percent natural abundance of deuterium is 0.015%. part a if it can be done with 100% efficiency, what mass of naturally occurring hydrogen gas would have to be processed to obtain a sample containing 2.50ã1021 2h atoms?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

You know the right answer?
Deuterium, 2h (2.0140 u), is sometimes used to replace the principal hydrogen isotope 1h in chemical...
Questions

Mathematics, 27.03.2020 19:18


Mathematics, 27.03.2020 19:18

Computers and Technology, 27.03.2020 19:18


Social Studies, 27.03.2020 19:18

Chemistry, 27.03.2020 19:18

Computers and Technology, 27.03.2020 19:18

English, 27.03.2020 19:18

Physics, 27.03.2020 19:18

Mathematics, 27.03.2020 19:18

Social Studies, 27.03.2020 19:18






Mathematics, 27.03.2020 19:18


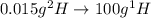
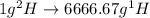
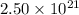 .
. 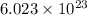 atoms thus,
atoms thus,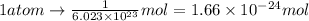
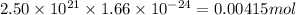
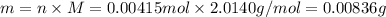
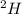 is 0.00836 g
is 0.00836 g