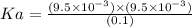Chemistry, 09.07.2019 04:30 ammullims822
In a 0.100 m hf solution, the percent dissociation is determined to be 9.5%. calculate the ka for hf based on this data.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 23.06.2019 05:40
Why is any chemical reaction always balanced? give reasons and explain the easiest way to solve the balancing problems in chemical equations with stoichiometric coefficients upto 20 as hit and trial doesn't always work. give full reasoning
Answers: 1

Chemistry, 23.06.2019 10:00
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1

Chemistry, 23.06.2019 13:30
Determine the osmotic pressure at 25 °c of an aqueous solution that is 0.028 m nano3. a) 0.685 atm b) 0.0729 atm c) 1.37 atm d) 0.0364 atm e) 2.06 atm
Answers: 2
You know the right answer?
In a 0.100 m hf solution, the percent dissociation is determined to be 9.5%. calculate the ka for hf...
Questions



Social Studies, 08.02.2021 16:30

Physics, 08.02.2021 16:30


Mathematics, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30

Mathematics, 08.02.2021 16:30




Biology, 08.02.2021 16:30


Mathematics, 08.02.2021 16:30

English, 08.02.2021 16:30



Mathematics, 08.02.2021 16:30


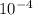
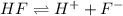
![Ka=\frac{[H^{+}][F^{-}]}{[HF]}](/tpl/images/0068/1234/53c4b.png) ............. (1)
............. (1)![[H^{+}]](/tpl/images/0068/1234/85507.png) by using the concentration and % dissociation.
by using the concentration and % dissociation.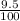 = 9.5 ×
= 9.5 × 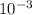 M
M![[F^{-}]](/tpl/images/0068/1234/3ebb8.png) , the concentration of
, the concentration of