
Chemistry, 09.07.2019 04:30 ryrytkg5107
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what would be the effect on the equilibrium position of removing cl2(g)? 1. reaction would go to the left, making more "reactants" 2. no change on the equilibrium position 3. reaction would go to the right, making more "reactants" 4. reaction would go to the left, making more "products" 5. reaction would go to the right, making more "products"

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
One of the cell membrane's functions is to protect the cell keep wastes in the cell create new cells keep light out of the cell
Answers: 1

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2
You know the right answer?
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what wou...
Questions

Computers and Technology, 11.12.2020 02:10

English, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

History, 11.12.2020 02:10


Mathematics, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

Physics, 11.12.2020 02:10



English, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10


Biology, 11.12.2020 02:10

Biology, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

Mathematics, 11.12.2020 02:10

Arts, 11.12.2020 02:10


English, 11.12.2020 02:10

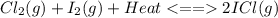
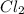 is removed from the system, the concentration of chlorine (
is removed from the system, the concentration of chlorine (

