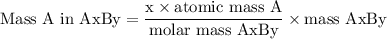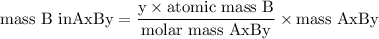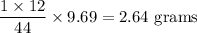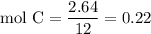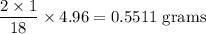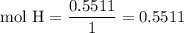Chemistry, 09.07.2019 07:30 kitttimothy55
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the empirical formula for the hydrocarbon?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 07:40
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2
You know the right answer?
Complete combustion of 3.20g of a hydrocarbon produced 9.69g of co2 and 4.96g of h2o. what is the em...
Questions



Mathematics, 19.05.2020 03:13


Mathematics, 19.05.2020 03:13

Arts, 19.05.2020 03:13




Health, 19.05.2020 03:13



Social Studies, 19.05.2020 03:13

Mathematics, 19.05.2020 03:13


Mathematics, 19.05.2020 03:13

Mathematics, 19.05.2020 03:13



Mathematics, 19.05.2020 03:13