
Chemistry, 09.07.2019 08:00 rlumanlan549
Asample of a smokestack emission was collected into a 1.25-l tank at 752 mm hg and analyzed. the analysis showed 92% co2, 3.6% no, 1.2% so2, and 4.1% h2o by mass. what is the partial pressure exerted by each gas?

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 2
You know the right answer?
Asample of a smokestack emission was collected into a 1.25-l tank at 752 mm hg and analyzed. the ana...
Questions

Mathematics, 19.11.2020 23:00



English, 19.11.2020 23:00

Mathematics, 19.11.2020 23:00

Mathematics, 19.11.2020 23:00



Computers and Technology, 19.11.2020 23:00

Chemistry, 19.11.2020 23:00


Mathematics, 19.11.2020 23:00

English, 19.11.2020 23:00

Mathematics, 19.11.2020 23:00

Mathematics, 19.11.2020 23:00

English, 19.11.2020 23:00

Mathematics, 19.11.2020 23:00


Mathematics, 19.11.2020 23:00

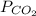 = 639.952 mmHg
= 639.952 mmHg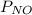 = 36.757 mmHg
= 36.757 mmHg = 5.757 mmHg
= 5.757 mmHg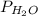 = 69.533 mmHg
= 69.533 mmHg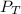 = 752 mmHg
= 752 mmHg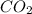 , NO,
, NO, 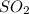 and
and 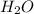 are 44, 30, 64, 18 respectively.
are 44, 30, 64, 18 respectively.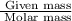 ........(1)
........(1)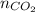 =
= 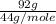 = 2.09 moles
= 2.09 moles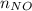 =
= 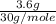 = 0.12 moles
= 0.12 moles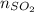 =
= 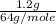 = 0.018 moles
= 0.018 moles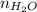 =
= 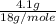 = 0.227 moles
= 0.227 moles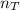 is calculated as
is calculated as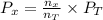 .........(2)
.........(2)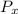 = partial pressure of x
= partial pressure of x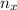 = moles of x
= moles of x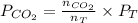 =
= 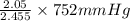 = 639.952 mmHg
= 639.952 mmHg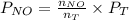 =
= 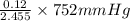 = 36.757 mmHg
= 36.757 mmHg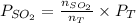 =
= 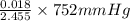 = 5.5136 mmHg
= 5.5136 mmHg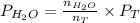 =
= 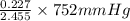 = 69.533 mmHg
= 69.533 mmHg

