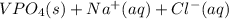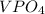Chemistry, 09.07.2019 08:00 aaronlikly
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rules to identify the composition of the salt that precipitates out of the solution. a. vpo4 b. na3po4 c. vcl3 d. nacl

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2



Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3
You know the right answer?
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rule...
Questions


Computers and Technology, 31.03.2021 20:50







Physics, 31.03.2021 20:50

Mathematics, 31.03.2021 20:50



Biology, 31.03.2021 20:50


Mathematics, 31.03.2021 20:50

Computers and Technology, 31.03.2021 20:50



Mathematics, 31.03.2021 20:50

Mathematics, 31.03.2021 20:50

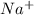 ,
,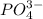 ,
,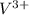 , and
, and 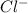 . The products formed in the reaction would be NaCl and VPO
. The products formed in the reaction would be NaCl and VPO . As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with
. As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with 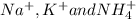 .
.