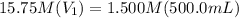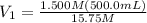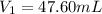Chemistry, 09.07.2019 13:30 genyjoannerubiera
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist with 10.00 l of 15.75 m perchloric acid solution to prepare the required solution. calculate the volume of concentrated acid required.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist wi...
Questions





Mathematics, 24.03.2021 17:30

History, 24.03.2021 17:30


Mathematics, 24.03.2021 17:30



Mathematics, 24.03.2021 17:30



History, 24.03.2021 17:30

Chemistry, 24.03.2021 17:30

Computers and Technology, 24.03.2021 17:30


Mathematics, 24.03.2021 17:30


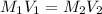
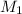 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and 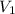 is it's volume.
is it's volume. 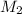 is the concentration of the diluted solution and
is the concentration of the diluted solution and 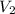 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for